Healthcare technologies in the 21 century

Technological development in every area of people’s lives significantly increases its pace. More than that overwhelming majority of organizations and companies tend to invest in investigations and own laboratories. The general characteristic of the innovation market can be described as a word “diverse”, but at the same time the diversity of technologies needs to be strongly regulated. As far as medical sector has widen the boundaries of emergency and development of innovation inside this specific field, the demand for international regulatory documents has increased. Today we will familiarize you with the latest updates in the international standardization sphere for medical industry.
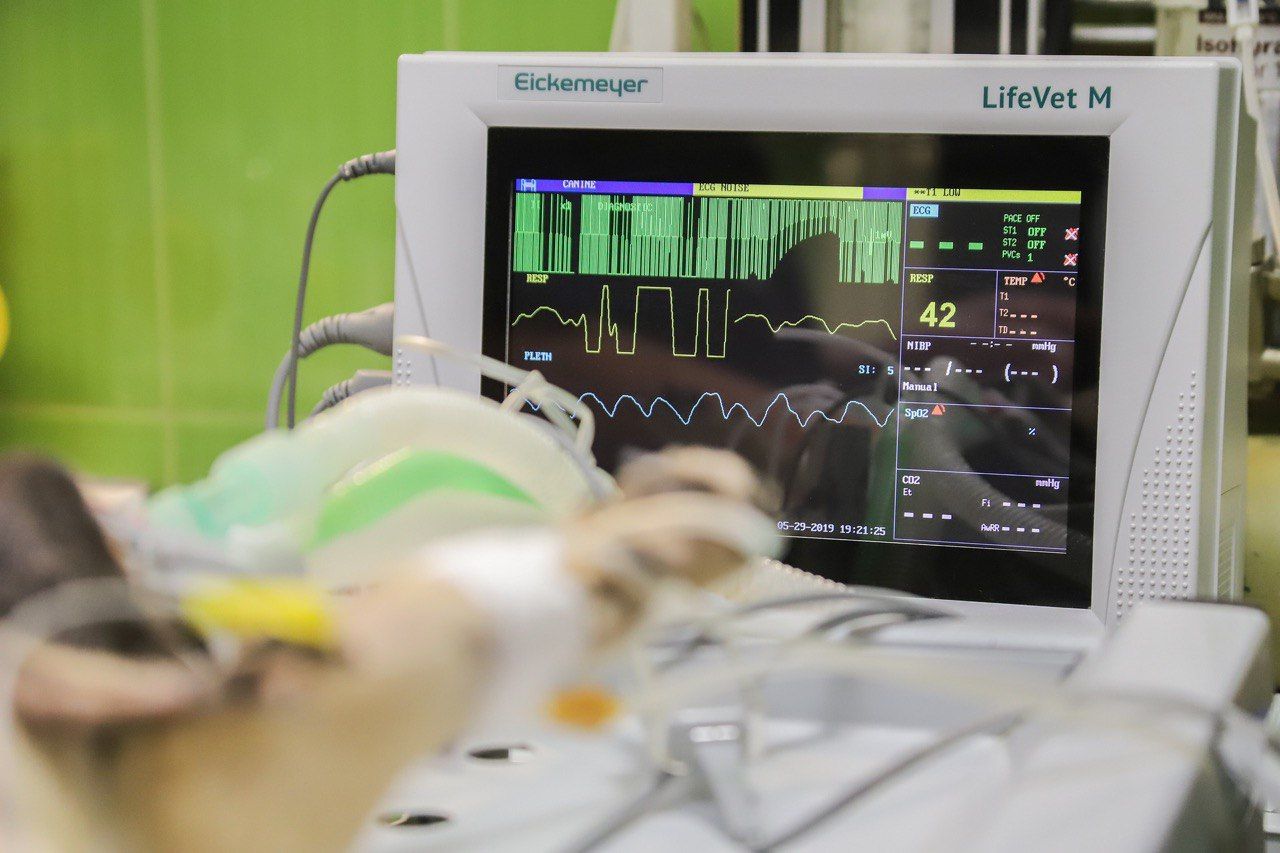
Health informatics - Standard communication protocol - Computer-assisted electrocardiography
Computer technologies are increasingly used in the daily life of every person. Since robotic technology greatly simplifies most of the actions carried out in different professions and also gives a better result, therefore, it is used in such an industry as medicine. One of the documents regulating its use is EN 1064: 2020.
This document specifies the common conventions required for the cart-to-host as well as cart-to-cart interchange of specific patient data (demographic, recording, ...), ECG signal data, ECG measurement and ECG interpretation results. This document specifies the content and structure of the information which is to be interchanged between digital ECG carts and computer ECG management systems, as well as other computer systems where ECG data can be stored
Despite belonging to the medical sector, this document has a technical focus, which is why we recommend that you familiarize yourself with its characteristics in more detail by clicking on the link to our website.
Health informatics - Device interoperability - Part 20701: Point-of-care medical device communication - Service oriented medical device exchange architecture and protocol binding (ISO/IEEE 11073-20701:2020)
A protocol for the use of medical devices can be prescribed even at such a global level as international standardization. Since today medical equipment has a different specific focus for operation, therefore, for each of these areas, various international regulatory documents are being created, one of which is EN ISO 11073-20701: 2020.
The scope of this standard is a service-oriented medical device architecture and communication protocol specification for distributed system of Point-of-Care (PoC) medical devices and medical IT systems that need to exchange data or safely control networked PoC medical devices. It identifies the functional components, their communication relationships as well as the binding of the components and communication relationships to protocol specifications.
If you have any questions about this standard, you can contact the iTech team, which will help clarify all aspects of your interest.
Health informatics - Device interoperability - Part 10201: Point-of-care medical device communication - Domain information model (ISO/IEEE 11073-10201:2020)
Communication models and the regulation of their implementation can be primarily associated with industries not related to medical activities. Nevertheless, today this type of technology has become quite popular and, due to its popularization, has undergone international regulation by such standards as EN ISO / IEEE 11073-10201: 2020.
The scope of this project is to define a general object-oriented information model that may be used to structure information and identify services used in point-of-care (POC) medical device communications. The scope is primarily focused on acute care medical devices and the communication of patient vital signs information.
If the activity of your organization comes into contact with the exchange of information and communication processes in the field of medicine, we recommend that you pay attention to this document.
Health informatics -- Requirements for international machine-readable coding of medicinal product package identifiers
Programming and computer science have significantly increased their influence on the introduction of innovative technologies in the medical sector. Moreover, there is an increasing need for qualified personnel in this industry. If it is quite specific, international standards such as ISO / TS 16791: 2020 are being created to simplify the implementation of technology and innovation in medicine.
This document provides guidelines on identification and labelling of medicinal products from the point of manufacture of packaged medicinal product to the point of dispensing the product. This document outlines best practice for AIDC barcoding solutions for applications. Users can, however, consider the coding interoperability requirements for other AIDC technologies, e.g. Radio Frequency Identification (RFID).
Due to the fact that this document is quite general and globalized, it can be applied in almost all organizations that cooperate with the medical sector or directly carry out activities in it.
Health Informatics - Requirements for a knowledge base for clinical decision support systems to be used in medication related processes (ISO/DTS 22756:2020)
ISO PRF/TS 22756:2020
For the implementation of certain processes in the field of medicine, it is necessary to have a high level of knowledge in the category in which the specialist operates. For the implementation of new medical devices and support systems, certain regulatory systems are being created. International standards such as ISO PRF / TS 22756: 2020 are one example of such systems.
This document specifies the requirements for developing a knowledge base for drug-related problems that cohere with the intended drug use, to be used in rule-based clinical decision support systems (CDSS), such as the criteria for selecting a raw data source and the quality criteria for the development and maintenance for the rules or clinical rules for drug safety. It also describes the process of how to develop a knowledge base, the topics to be considered by the developers of a knowledge base, and it gives guidance on how to do this. This document gives guidelines for the development of a knowledge base:
- with rules to enhance decisions and actions in drug-related problems that cohere with the intended drug use;
- which can be used by all kinds of healthcare professionals, such as those who prescribe, dispense, administer or monitor medicines;
- which can be used in every care setting, including chronic and acute care, primary and specialized care;
- which is a repository of evidence/practice bases rules, assessed by experts;
- which is meant to be used in conjunction with a medicinal product dictionary;
- whose knowledge is structured in rules and therefore to be used in the type of rule-based CDSS. This document does not:
- describe the exact content of a knowledge base i.e. the outcome of the process of developing rules.
- provide the requirements for a clinical decision support system, the software that uses the knowledge base combined with the patient's data, and presents the outcome of the rules to the healthcare professional. These requirements are described in ISO/DTS 22703[1].
- give the requirements for non-medication knowledge bases. Some aspects of the requirements in this document are general in nature and applicable to other kinds of knowledge bases, but this document does not address all of the requirements of non-medication knowledge bases.
If you are interested in purchasing this document, we recommend that you clarify all the technical characteristics as well as details of possible areas of its application in order to avoid mistakes in choosing the standard necessary to increase the productivity of your organization.
Cable networks for television signals, sound signals and interactive services - Part 2-4: Interference Mitigation Filters operating in the 700 MHz and 800 MHz bands for DTT reception
Cable technology and electricity transmission networks are primarily associated with the energy industry. Nevertheless, these technologies are actively used in the field of medicine and even international standards are being created for them. One of the documents regulating the use and implementation of this technology in medicine is EN 50083-2-4: 2019.
This document provides requirements for passive filters intended to reduce RF interference from mobile Base Stations (BS) and User Equipment (UE) to receiving equipment and master antenna cable distribution systems of broadcast DVB-T and DVB-T2 signals in the VHF and UHF bands. While primarily intended to be used with VHF/UHF DVB-T and DVB-T2 receivers and signal distribution systems, filters can also be useful for mitigation of interference to VHF FM or DAB radio.
Since this document has clear technical parameters that you can check with the technologies used by you, we recommend that you study these aspects in more detail in order to avoid the risks of using the wrong document in your field of activity.
Cable networks for television signals, sound Signals and interactive services - Part 2-3: LTE (4G) Interference Mitigation Filters
Since computer technology and cable networks, like any other industry, has a large number of subcategories, therefore, the number of international standards is appropriate. One of the following documents regulating the aforementioned areas of innovation is CLC / TS 50083-2-3: 2018.
This Technical Specification provides requirements to passive filters intended to reduce RF interference from LTE Base Stations (LTE-BS) and LTE User Equipment (LTE-UE) to receiving equipment and cable distribution systems of broadcast DVB-T and DVB-T2 signals in the VHF and UHF bands. While primarily intended to be used with VHF/UHF DVB-T and DVB-T2 receivers and signal distribution systems, filters can also be useful for mitigation of interference to VHF FM or DAB radio.
In order to avoid confusion about the large variation of similar technologies in the medical sector, we recommend that you study the technical parameters and characteristics of the standard in more detail and also compare them with those already existing in your organization.
The bigger variety - the stronger control
As we can see, in the medical sector a wide variety of technologies is used. Since this area is distinguished by being responsible for human lives, therefore, the level of control over the introduction of new technologies, as well as the process of use, must be carried out in the most serious way. If your organization comes into contact with the use or production of medical equipment, you must be aware that, first of all, you are responsible not only for the financial results of your business activities, but also social responsibility for people's lives. That is why the availability of international standards for this kind of companies and organizations is necessary, not only for successful business activities, which in turn is a competitive advantage, but also for confidence in the complete safety of technology operation.
References:
https://standards.iteh.ai/catalog/standards/cen/4155b086-378f-4431-b970-6a038f6fbefd/en-1064-2020
Categories
- Latest News
- New Arrivals
- Generalities
- Services and Management
- Health Care
- Environment
- Metrology and Measurement
- Testing
- Mechanical Systems
- Manufacturing
- Electrical Engineering
- Electronics
- Telecommunications
- Information Technology
- Road Vehicles
- Railway Engineering
- Materials Handling
- Agriculture
- Food technology
- Petroleum
- Metallurgy
- Wood technology
- Construction
- Entertainment